Percutaneous Mechanical Circulatory Support Devices Market to Hit $5.20B by 2033 | DataM Intelligence Report 2025-2033
The Percutaneous Mechanical Circulatory Support Devices Market is projected to grow steadily, reaching $5.20B by 2033, driven by rising cardiac cases and Tech
The U.S. Percutaneous Mechanical Circulatory Support Devices Market is driven by rising heart failure cases, contributing significantly to the projected global market value of USD 5.20B by 2033.
”
AUSTIN, TX, UNITED STATES, June 17, 2025 /EINPresswire.com/ -- Market Growth & Value Outlook 2025-2033— DataM Intelligence
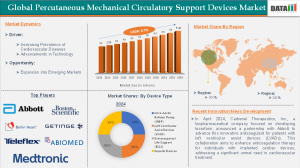
The Percutaneous Mechanical Circulatory Support Devices Market was valued at USD 2.35 Billion in 2024 and is expected to rise to approximately USD 5.20 Billion by 2033, growing at a CAGR of 8.7% between 2025 and 2033.
Moreover, increasing awareness about early-stage cardiac care and the introduction of more compact and efficient devices are prompting healthcare facilities to adopt pMCS devices more actively, especially in critical care and emergency units.
To Download Sample Report:https://www.datamintelligence.com/download-sample/percutaneous-mechanical-circulatory-support-devices-market
Regional Analysis
North America
North America, especially the United States, holds the largest market share owing to a well-developed healthcare infrastructure, high healthcare spending, and early adoption of advanced technologies. Strong backing from government and private insurance also contributes to the rapid adoption of pMCS devices across hospitals.
Europe
Countries like Germany, France, and the UK are showing steady market penetration due to an aging population and increased prevalence of cardiovascular ailments. EU-based companies are focusing on product innovation to gain competitive advantage.
Asia-Pacific
The Asia-Pacific region is projected to experience the most rapid growth throughout the forecast period. Nations like Japan, China, and India are investing in modernizing their healthcare infrastructure and expanding access to advanced cardiac care. Japan, in particular, stands out due to its strong presence in medical technology development and regulatory support for innovation.
Latin America & MEA
Though these regions are at nascent stages of adoption, growing awareness and international partnerships are slowly driving the market forward. Countries like Brazil and Saudi Arabia are emerging as key markets with strong growth potential.
Competitive Landscape
Key companies include:
Abbott
Berlin Heart
Medtronic
Teleflex Incorporated.
Getinge
ABIOMED.
Boston Scientific Corporation
LivaNova, Inc.
EUROSETS
Evaheart, Inc.
Market Segmentation:
By product: Va-Extracorporeal Membrane Oxygenation (ECMO), Intra-Aortic Balloon Pumps, Short Term Ventricular Assist Devices, Impella, Tandemheart, Others
By End-User: Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Others
By Region: North America, Latin America, Europe, Asia Pacific, Middle East, and Africa
Industry Developments:
In April 2024, Cadrenal Therapeutics, Inc., a biopharma company developing tecarfarin, entered into a partnership with Abbott to advance this novel anticoagulant for patients using left ventricular assist devices (LVADs). The collaboration seeks to improve anticoagulation therapy for those with implanted heart devices, targeting a critical gap in cardiovascular care.
Latest News from the USA
In Q2 2025, Abiomed, under Johnson & Johnson MedTech, introduced the next-generation Impella ECP (Expandable Cardiac Power) device designed to improve heart recovery in critical patients. This ultra-small, fully percutaneous heart pump was developed for high-risk PCI and cardiogenic shock cases. The product gained FDA Breakthrough Device status, reinforcing its potential in saving lives with minimally invasive technology.
Meanwhile, several hospitals across the U.S., including Mayo Clinic and Cleveland Clinic, reported improved survival rates and shorter ICU stays among patients supported by percutaneous mechanical pumps during high-risk interventions. These encouraging results are further pushing adoption in cardiac units.
Additionally, in April 2025, the American College of Cardiology (ACC) published new guidelines highlighting the use of pMCS devices in the management of acute decompensated heart failure and post-MI complications, validating their importance in clinical protocols.
Latest News from Japan
Japan has been actively promoting innovation in cardiac support technologies. In May 2025, Terumo Corporation unveiled a collaborative research project with Tokyo University Hospital focused on developing smart percutaneous circulatory support devices powered by AI-based flow modulation. This aims to personalize circulatory support in real time based on patient-specific physiological data.
Regulatory bodies in Japan have also simplified approval pathways for novel cardiac technologies under the “Fast-Track Medical Device Review Program, encouraging international companies to bring advanced pMCS solutions to the Japanese market.
Moreover, the Japanese Society of Interventional Cardiology (JSIC) hosted a special workshop in June 2025 discussing the long-term benefits of short-duration mechanical support for patients undergoing high-risk PCI, which has led to wider training initiatives for cardiologists across the country.
Future Outlook
The percutaneous mechanical circulatory support devices market is set to grow rapidly in the coming years, fueled by clinical validation, evolving guidelines, and rising demand for minimally invasive treatment options. While the U.S. leads in technology adoption and clinical application, Japan is setting benchmarks in innovation and regulatory agility.
As cardiac care becomes increasingly personalized, future pMCS devices are likely to integrate real-time data analytics, wireless monitoring, and biocompatible materials, marking the next wave of transformation in heart failure management and interventional cardiology.
Here are the Recent Related Reports By DataM intelligence:
Tympanostomy Products Market Size 2025-2033
Carotid Stenotic Scan Devices Market Size By 2031
Sai Kiran
DataM Intelligence 4Market Research
+1 877-441-4866
Sai.k@datamintelligence.com
Visit us on social media:
LinkedIn
X
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.